GENERAL MECHANISM OF ACTION OF INSULIN AND RELATE HORMONES
- The body's main fuel is Glucose. Glucose is stored in the liver and muscles as Glycogen.
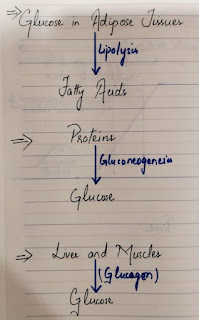
- Excess glucose is stored in the adipose tissues which yield fatty acids via lipolysis. In the fasting state, free fatty acids supply much of the energy needs of the body except for the CNS which requires Glucose to function normally.
- Proteins can also be converted to glucose via gluconeogenesis.
RELEASE OF INSULIN AND GLUCAGON
- Insulin and Glucagon are produced in the pancreas by the Islets of Langerhans. Beta cells make up 70%-90% of the Islets and produce Insulin and Amylin while the a-cells produce glucagon.
- The main function of insulin is to decrease blood glucose levels.
- Glucagon, along with other counter-regulatory hormones such as growth factor, cortisol, and Epinephrine, increases Blood glucose levels.
- The opposing actions of Glucagon and Insulin along with other counter-regulatory hormones maintain a normal fasting values of 79-99mg/dL.
FUNCTION OF INSULIN/ NORMAL INSULIN ACTION
After the food is consumed, blood glucose levels rise, and the secretion of insulin occurs in two phases:
Phase 1-FIRST PHASE INSULIN RESPONSE:
An initial burst lasts approx. 5-10 minutes and serves to suppress hepatic glucose production and causes insulin-mediated glucose disposal in adipose tissues. This bolus of insulin minimizes hyperglycemia during meals and during post-prandial period.
Phase 2:
The second phase is characterized by the gradual increase in insulin secretion, which lasts 60-120 minutes and stimulates the glucose uptake by Peripheral insulin dependent tissues.
FUNCTION OF AMYLIN
- Amylin is the naturally occurring hormone that is co-secreted by the beta cells along with Insulin.
- People with diabetes either have a relative or complete lack of amylin.
- 3 major functions of Amylin are:
- a) Suppression of post meal glucagon secretion
- b) Regulation of the rate of gastric emptying from the stomach to the small intestine, which increases satiety.
- c)Regulation of plasma glucose concentrations in the bloodstream.


Comments
Post a Comment